 Do you have a question you would like to Ask The Sietch? Contact us or post your question in the Forums, and we will do our best to answer it.
Do you have a question you would like to Ask The Sietch? Contact us or post your question in the Forums, and we will do our best to answer it.
A while ago in a post about making ethanol from orange pulp Mr Clint asked:
Tell us how to make it..please?
A lot of people ask me things like “just how is ethanol made anyway?” So lets talk a little about ethanol, how its made, and how you would go about making it at home if you wanted to. First what is ethanol anyway?
What is ethanol?
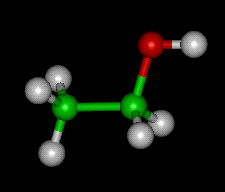
The chemical compound ethanol. Ethanol's chemical formula is C2H5OH
Ethanol also known as grain alcohol or ethyl alcohol, can be produced chemically from ethylene or biologically from the fermentation of various sugars from carbohydrates found in agricultural crops and cellulosic residues from crops or wood. When non-chemists refer to “alcohol”, they almost always mean ethanol.
You take something with a carbohydrate (sugar usually) and let various microorganisms go to town on it, the little beasties eat up the carbs and poop out alcohol. Most current methods of creating ethanol also require distilation to remove impurities from the alcohol. This is a similar process to the one that makes beer, wine, whiskey, hard cider, and some acids.
Pure ethanol is a flammable, colorless liquid with a boiling point of 78.5° C. Its low melting point of -114.5° C allows it to be used in antifreeze products. It has a pleasant odor reminiscent of whiskey.
Its density is 789 g/L, about 20% less than that of water. It is easily soluble in water and is itself a good solvent.
Ethanol can lose a proton from the hydroxyl group and is a very weak acid, weaker than water.
The CAS number of ethanol is 64-17-5 and its UN number is UN 1170.
If you were interested here is the equation for glucose (the simplest sugar) to ethanol.
Chemical Equation
C6H12O6 → 2C2H5OH + 2CO2 + 2 ATP (Energy Released:118 kJ mol^−1)
Word Equation
Sugar (glucose, fructose, or sucrose) → Alcohol (ethanol) + Carbon Dioxide + Energy (ATP)
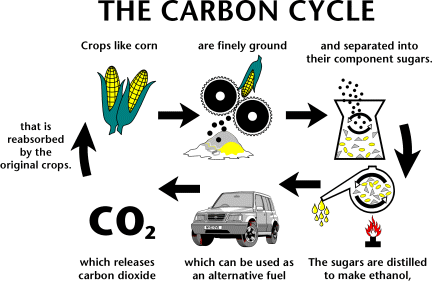
As you can see when ethanol is made it releases co2. However this is the same co2 that future plants will be taking in to grow. So in a perfect world ethanol is carbon neutral (it doesn’t add more carbon to the atmosphere). There is however a large debate over weather or not the entire process of creating ethanol (growing the plants, shipping, fermenting, distilling etc) is carbon neutral.
What can you use ethanol for?
So now we know what ethanol is, lets talk a bit about what it is used for. Ethanol can be used as a solvent in perfumes, paints and tinctures. The use most people are familiar with is when they kick back a bear or a shot at the bar. The various tastes of different drinks are because the ethanol absorbs different amounts of various impurities giving the drink its special flavor and kick.
A solution of 70-85% of ethanol is commonly used as a disinfectant; it kills organisms by denaturing their proteins and dissolving their lipids: it is effective against most bacteria and fungi, and many viruses, but is ineffective against bacterial spores. This disinfectant property of ethanol is one of the reasons that alcoholic beverages can be stored for a long time. Ethanol is also used to preserve tissue and specimens due to its protection against bacteria and fungi.
Ethanol has also been gaining a lot of attention lately as a fuel source.
Ethanol as a fuel:
Most cars since the late 80’s have been manufactured to use some amount of ethanol. Due to its solvent nature special hoses and gaskets must be installed in a car to allow it to use ethanol. Almost all cars will run on a 10% blend (90% gasoline and 10% ethanol sometimes called gasohol) of ethanol and many new cars are “E85” ready. Meaning they can run on an 85% ethanol and 15% gasoline. These cars are often called duel fuel, flex fuel, or biofuel ready cars. Due to the higher octane content of ethanol you will also need a car that can adjust its spark plug timing. Flex fuel cars and trucks are able to detect what fuel is put into them and adjust the timing as needed.
What are the environmental benefits of using ethanol?
- It is a renewable fuel made from plants
- It is not a fossil-fuel: manufacturing it and burning it does not increase the greenhouse effect
- It provides high octane at low cost as an alternative to harmful fuel additives
- Ethanol blends can be used in all petrol engines without modifications
- Ethanol is biodegradable without harmful effects on the environment
- It significantly reduces harmful exhaust emissions
- Ethanol’s high oxygen content reduces carbon monoxide levels more than any other oxygenate: by 25-30%, according to the US EPA
- Ethanol blends dramatically reduce emissions of hydrocarbons, a major contributor to the depletion of the ozone layer
- High-level ethanol blends reduce nitrogen oxide emissions by up to 20%
- Ethanol can reduce net carbon dioxide emissions by up to 100% on a full life-cycle basis
- High-level ethanol blends can reduce emissions of Volatile Organic Compounds (VOCs) by 30% or more (VOCs are major sources of ground-level ozone formation)
- As an octane enhancer, ethanol can cut emissions of cancer-causing benzene and butadiene by more than 50%
- Sulphur dioxide and Particulate Matter (PM) emissions are significantly decreased with ethanol.
(via)
So now that we know what ethanol is and how we use it how would we go about making it at home.
How to make ethanol at home:
Ever hear of moonshine? How about back yard stills? The technology to make ethanol has been around for a long, long time. The only difference between a moonshine still and an ethanol still is the proof of the resulting ethanol. Ethanol needs to have a very high proof (180+) in order to function as a fuel. This means that you will need to distill almost all of the impurities out of the ethanol to use it as a fuel.
The easiest way to start making ethanol is to buy or make your own still. A still is a device that allows you to ferment and distill ethanol from a feed stock. The feed stock can be sugar, corn, or anything else that has a carbohydrate in it. Some newer forms of ethanol are made using cellulose feed stocks. They use special bacteria that can break down cellulose into sugars and then they ferment these sugars into alcohol. Some have even figured out bacteria that will break the cellulose down into alcohol taking out the fermenting stage and saving a lot of energy.
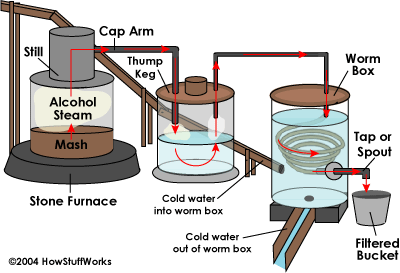
This is a diagram of a moonshine still, an ethanol still works in much the same way. In essence you heat up the “mash” (fermented corn, sugar, whatever) and that begins producing alcohol vapor(it boils the alcohol out of the mash), the vapor is captured and cooled and forced to condense into liquid alcohol. You would then remove impurities and water from this alcohol to create fuel grade ethanol. Read more about ethanol creation here (pdf)
Here are some places you can buy sell contained ethanol stills (I make no guarantee any of these places are legit, but some look better than others).
- Ethanol Stills
- Running on alcohol
- Ethanol Pro Moonshine Stills
- Cowboy energy
- Copper moonshine stills
You can also find DIY instructions here Also Journey To Forever has an amazing ethanol (and other biofuel) resources, from DIY stills, to instructions on how to make your own mash to how to distill the final product. They are really a great resource check them out here. You can also read about some others experience with home made ethanol here.
I hope this helps you on your way to making your own ethanol at home. Good luck.
Edit: Thanks Joel for help with the fermenting stage.

Er…to my understanding, the fermenting of the mash is already done by the time distillation begins. At the temperature that alcohol boils, I believe the yeast isn’t active…it might even be dead.
Sometimes part of the spent mash is added back to a fresh fermentation, so that the acetic acid in it can cut back on bacterial growth, which might be what you were thinking of.
And while you might not want to introduce the “azeotrope,” I think it’s also worth mentioning that distillation alone can only achieve 96% purity. Removing the last bit of water from one’s fuel means mixing in some glycerol or petrochemicals before the last distillation.
Joel: You are correct, the fermentation is done before the distillation takes place, I re-read the article and could see how the way I wrote it would make someone think that, it will be fix thanks :)
As for needing an additive to get the final purity, if you are making biodiesel as well as ethanol you should have plenty of glycerin left over from that process to feed into your ethanol making. I wonder if the two could be combined in some way, perhaps make cellulitic ethanol from the waste husks of the soybeans and other oil plants you use to make the biodiesel?
Thanks for the comments!
Yes, indeed. I was poring over the Wikipedia article on glycerine just a few weeks ago, and found this excellent how-to link on making absolute alcohol here. After re-reading it, I remembered that the method the author suggests distilling a potassium carbonate-in-glycerine solution to remove all the water, and bubbling the alcohol vapor through this before condensing it.
There are lots of helpful hints for the various types of still that were available in 1924.
What does UN 1170 stand for? literally?
Jim: UN numbers are a code to describe chemcials here is more info http://en.wikipedia.org/wiki/UN_number it helps with shipping and things like that. They are assigned by the United Nations, hence, UN.
Hope that helps.
Hey guys… I don’t know much about ethanol but i had to do a project on BIOETHANOL, whts the difference?????????????? Because im a bit confused… I love this website!!! Naib and all the other guys: You are so clever… This is very interesant, please answer my question, ill b grateful if you did… Eager+waiting…
Hi Ashley, bioethanol is made from organic sources, as opposed to fossil fuel sources. That is how the word is usually used.
Hope this helps.
Naib, thanks so much, that did help, i apreciate your anser, thanks again…
Ok maybe you think im annoying but im just curious: What is ethanol’s ph??? just curious…
and this is what a friend told me something like this, this is what i can remember…
”If ethanol’s so good, why does it need government subsidies? Shouldn’t producers be eager to make it, knowing that thrilled consumers will reward them with profits? But consumers won’t reward them, because without subsidies, ethanol would cost much more than gasoline. The claim that using ethanol will save energy is another myth. Studies show that the amount of energy ethanol produces and the amount needed to make it are roughly the same. It takes a lot of fossil fuels to make the fertilizer, to run the tractor, to build the silo, to get that corn to a processing plant, to run the processing plant… And because ethanol degrades, it can’t be moved in pipelines the way that gasoline is. So many more big, polluting trucks will be needed to haul it. More bad news: The increased push for ethanol has already led to a sharp increase in corn growing — which means much more land must be plowed. That means much more fertilizer, more water used on farms and more pesticides. ”
Ashley: I would say all but the government subsides part are valid problems with ethanol. Sometimes new technologies need to be subsidized, and its not like oil companies don’t get billions in subsides every year.
I personally think that ethanol is a dead end technology unless it can be made from sources that are not food, and made locally. I think that we should be moving towards electric cars powered with solar and wind. I think your friend is right on most accounts.
All that being said, advances in technology may allow for cheap, non fertilized local, non-food supplies of ethanol to be produced.
Thanks, I’ll tell her. I think everyone should try to pollute less and try to save energy… I studied bioethanol which had many links, one example that I found out is that some people in Mexico can’t afford buying tortillas. (which are some type of omlet made out of corn and bread) This is beacuse corn prices in the US are rising and Mexico imports corn from them. A large amount of corn in the US is used to make bioethanol and I also found out its (US) bioethanol production has increased so much that it has already a bigger production than Brazil’s. Well the point is: if corn from the US is used for bioethanol production, Mexico has less corn when imported from the US, this means corn prices in Mexico will rise because there is less corn, and this means people can’t afford tortillas. (which are their staple diet) I personaly think Mexico should grow corn and not import because if international prices go up, when the country imports it is very expensive. So bioethanol affects in many ways: in good ways and in bad ways. Bioethanol is being really useful and is used in many transports in some countries. (but many people still prefer oil and other fossil fuels) What I still doubt is if ethanol is a renewable energy… If i may know: what do YOU know about bioethanol? andwhat do you think about all this… and global warming, and al gores ideas… Im quite interested in this topic but I don’t want to be annoying either… By the way we managed to create ethanol in our school’s science lab.
… and … I think about ths and would like to know if im right: ”Although the raw materials utilized in an ethanol plant can come mainly (but not exclusively) from agricultural sources, ethanol production is an industrial process. Like all industrial processes, emissions are a fact of life around an ethanol facility. In addition to emissions, the noise levels, increased traffic, effects on watersheds, glare from lighting, effects on wildlife and so on, all have their impact on the surrounding community.”
I’d also like to know if im right thinking this: ”Although the raw materials utilized in an ethanol plant can come mainly (but not exclusively) from agricultural sources, ethanol production is an industrial process. Like all industrial processes, emissions are a fact of life around an ethanol facility. In addition to emissions, the noise levels, increased traffic, effects on watersheds, glare from lighting, effects on wildlife and so on, all have their impact on the surrounding community.”
i am doing a project on biodiesel vs. ethanol and i need help
what help do you need Felicia?
This project is due after Christmas because it’s quite loong and i alread have 92 pgs (I’m not even finished), we also have to write our personal opinion and I’d like to write this in but I don’t really know if I’m right thinking it: what do you think?:â€Although the raw materials utilized in an ethanol plant can come mainly (but not exclusively) from agricultural sources, ethanol production is an industrial process. Like all industrial processes, emissions are a fact of life around an ethanol facility. In addition to emissions, the noise levels, increased traffic, effects on watersheds, glare from lighting, effects on wildlife and so on, all have their impact on the surrounding community.†I’ll be really grateful if any of you guys could answer me and maybe some more stuff… :(
Hello Ashley: Every act has impacts. If you seem to be finding more negative aspects to ethanol than you are finding positive ones then perhaps ethanol is not worth it. However factories can always be built in locations that minimize effects on people living around them, you would be surprised what some trees, and earth berms and specially designed lights can accomplish to hide a factory.
Personally I think wind and solar are much better options, combined with electric cars. I think ethanol and bio-diesel are just band aids that are going to get us to that point.
Thank you so much, I’ll add some more things to it… Thanks for your help… Having looked at everyting, in my opinion I agree with you on that point, thnks… =)
I would like to ask specifically how ethynol is a cheaper fuel. My husband says it costs more money for the energy it takes to produce the product. It costs money for the fuel for the machine to pick the corn and costs money for the tires, it costs money to transport the corn to the plant using fuel again, and it costs money to heat and cause distillation. Then a truck has to transport the ethynol to a refinery and then a gas station. The refinery needs fuel to run it. How can this be cheaper?
Marianne: The hard answer might be that it isn’t cheaper. Which is something not a lot of people want to hear. Ethanol may turn out to be a very very bad way to go.
To Ashley re. Mexico Corn Imports,
Mexican farmers used to grow their own corn until NAFTA. Then their government was forced to lower import tariffs on US (subsidized)corn. The small farmers couldn’t compete with big US agro business and went north looking for jobs. That’s free trade for you.
i think it’s BS that we are goin to sit around, awine about how expensive ethanol is to make when we make it ALL the time for our tummies instead of our cars. i think that we need to get with a brewing company and ask the experts if we could make a super-cheap mash, and use it for distillation. if everybody put down their drinks, and put it in their car for one year, how much more would we have? we have many more options to consider than we let ourselves think we do. i think we have established that it is no longer a question of if, but when we run out of oil… what are we going to do? if we dont find out, what will our children, and grandchildren do? pay a million dollars for a gallon of gas?
Hi Loren
when was the last time you drank 26 gallons of alcohol. Cars use so much more fuel than humans do that there is no way we could “get with brewers” and solve this problem. The real solution to our fuel issues is to use less, and eventually stop using it all together. I support electric cars powered by wind/solar/geothermal…
Saya coba lakukan penggunaan etanol pa sepeda motor 2 stroke saya dengan menggunakann campuran 90% denfan memperkecil veturi dan hasilnya sangat baik
Your thumper is wrong. The pipe with the steam coming from the still is supposed to go to the bottom of the thumper so the steam bubbles up through the wash/water. The way your picture is, the steam would go straight in one pipe and out the other and nothing would happen to it.
Good Call Jim. I just looked at that diagram this morning for the first time and I agree with you. I am surprised there have not been similar comments on this.