One of the biggest problems with electric cars, is that the unreasonable and unnecessary demand by drivers that electric cars should be able to travel as far as gas cars on a single charge (even though most people never drive more than 50 miles in a day). The battery is the critical point in this scenario, better batteries mean longer distances traveled between charges, which means people will move en mass to the electric car. Lucky for us the eggheads over at Stanford have found a way to use silicon nanowires to reinvent the rechargeable lithium-ion battery. Not only will this greatly help electric cars, but has the potential to revolutionize laptops, iPods, video cameras, cell phones, and countless other devices.
The new version, developed through research led by Yi Cui, assistant professor of materials science and engineering, produces 10 times the amount of electricity of existing lithium-ion (Li-ion) batteries. A laptop that now runs on battery for two hours could operate for 20 hours, a boon to ocean-hopping business travelers.
“It’s not a small improvement,” Cui said. “It’s a revolutionary development. Given the mature infrastructure behind silicon, this new technology can be pushed to real life quickly,” Cui said.
The electrical storage capacity of a Li-ion battery is limited by how much lithium can be held in the battery’s anode, which is typically made of carbon. Silicon has a much higher capacity than carbon, but also has a drawback.
Silicon placed in a battery swells as it absorbs positively charged lithium atoms during charging, then shrinks during use (i.e., when rocking out to your Ipod) as the lithium is drawn out of the silicon. This expand/shrink cycle typically causes the silicon (often in the form of particles or a thin film) to pulverize, degrading the performance of the battery.
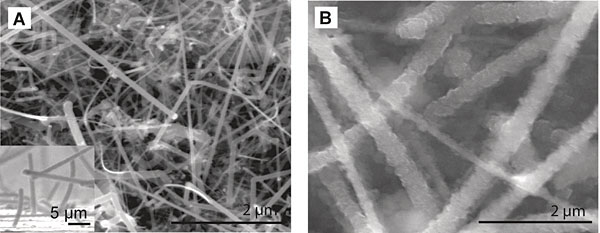
Cui’s battery gets around this problem with nanotechnology. The lithium is stored in a forest of tiny silicon nanowires, each with a diameter one-thousandth the thickness of a sheet of paper. The nanowires inflate four times their normal size as they soak up lithium. But, unlike other silicon shapes, they do not fracture.
Research on silicon in batteries began three decades ago. Chan explained: “The people kind of gave up on it because the capacity wasn’t high enough and the cycle life wasn’t good enough. And it was just because of the shape they were using. It was just too big, and they couldn’t undergo the volume changes.”
Then, along came silicon nanowires. “We just kind of put them together,” Chan said.
For their experiments, Chan grew the nanowires on a stainless steel substrate, providing an excellent electrical connection. “It was a fantastic moment when Candace told me it was working,” Cui said.
Cui said that a patent application has been filed. He is considering formation of a company or an agreement with a battery manufacturer. Manufacturing the nanowire batteries would require “one or two different steps, but the process can certainly be scaled up,” he added. “It’s a well understood process.”
I am eagerly awaiting these new batteries, as really there is nothing left to perfect. Electric motors are already far far superior to internal combustion engines, the rest is pretty much the same. If you could get an electric car that ran for 200-300 miles on a single charge would you buy one?
Unfortunately , no one of the dozen or so battery experts polled believes that Cui has done anything other than fool himself into believing that he has discovered something. He has shown zero results and none of those in the know think he ever will The numbers simply don’t make any sense.
200-300 miles? Tesla already did that. I would like to see 500-1000 mile range.