I have some pretty strong reservations about the mythical “hydrogen economy” mostly because of the way the hydrogen is made. For a successful hydrogen economy we will have to be able to make hydrogen out of waste material (not fossil fuel), and make it locally. Which is why I found it pretty interesting that by adding a few modifications to their successful wastewater fuel cell, researchers have been able to coax common bacteria to produce hydrogen in a new, efficient way.
Bruce Logan and colleagues at Penn State University had already shown success at using microbes to produce electricity. Now, using starter material that could theoretically be sourced from a salad bar, the researchers have coaxed those same microbes to generate hydrogen.
By tweaking their design, improving conditions for the bacteria, and adding a small jolt of electricity, they increased the hydrogen yield to a new record for this type of system.
“We achieved the highest hydrogen yields ever obtained with this approach from different sources of organic matter, such as yields of 91 percent using vinegar (acetic acid) and 68 percent using cellulose,” said Logan.
In certain configurations, nearly all of the hydrogen contained in the molecules of source material was converted to useable hydrogen gas, an efficiency that could eventually open the door to bacterial hydrogen production on a larger scale.
“Bruce Logan is a clear leader in this area of research on sustainable energy,” said Bruce Hamilton, National Science Foundation director of the environmental sustainability program at NSF and the officer overseeing Logan’s research grant. “Advances in sustainable energy capabilities are of paramount importance to our nation’s security and economic well-being. We have been supporting his cutting-edge research on microbial fuel cells for a number of years and it is wonderful to see the outstanding results that he continues to produce.”
Other systems produce hydrogen on a larger scale, but few if any match the new system for energy efficiency.
Even with the small amount of electricity applied, the hydrogen ultimately provides more energy as a fuel than the electricity needed to drive the reactor. Incorporating all energy inputs and outputs, the overall efficiency of the vinegar-fueled system is better than 80 percent, far better than the efficiency for generation of the leading alternative fuel, ethanol.
Even most electrolysis techniques, methods to extract hydrogen from water using electricity, pale in comparison to the new method.
“We can do that by using the bacteria to efficiently extract energy from the organic matter,” said Logan. By perfecting the environment for the bacteria to do what they already do in nature, the new approach can be three to ten times more efficient than standard electrolysis.
“The energy focus is currently on ethanol as a fuel, but economical ethanol from cellulose is 10 years down the road,” says Logan, the Kappe professor of environmental engineering. “First you need to break cellulose down to sugars and then bacteria can convert them to ethanol.”
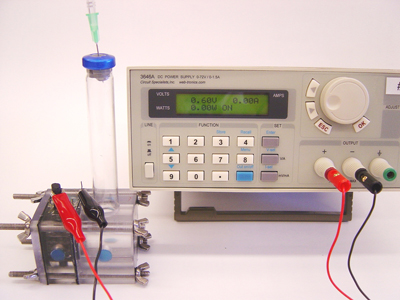
Logan and Shaoan Cheng, used naturally occurring bacteria in a microbial electrolysis cell with acetic acid – the acid found in vinegar. Acetic acid is also the predominant acid produced by fermentation of glucose or cellulose. The anode was granulated graphite, the cathode was carbon with a platinum catalyst, and they used an off-the-shelf anion exchange membrane. The bacteria consume the acetic acid and release electrons and protons creating up to 0.3 volts. When more than 0.2 volts are added from an outside source, hydrogen gas bubbles up from the liquid.
“This process produces 288 percent more energy in hydrogen than the electrical energy that is added to the process,” says Logan.
Water hydrolysis, a standard method for producing hydrogen, is only 50 to 70 percent efficient. Even if the microbial electrolysis cell process is set up to bleed off some of the hydrogen to produce the added energy boost needed to sustain hydrogen production, the process still creates 144 percent more available energy than the electrical energy used to produce it.
For those who think that a hydrogen economy is far in the future, Logan suggests that hydrogen produced from cellulose and other renewable organic materials could be blended with natural gas for use in natural gas vehicles.
“We drive a lot of vehicles on natural gas already. Natural gas is essentially methane,” says Logan. “Methane burns fairly cleanly, but if we add hydrogen, it burns even more cleanly and works fine in existing natural gas combustion vehicles.”
The range of efficiencies of hydrogen production based on electrical energy and energy in a variety of organic substances is between 63 and 82 percent. Both lactic acid and acetic acid achieve 82 percent, while unpretreated cellulose is 63 percent efficient. Glucose is 64 percent efficient.
Another potential use for microbial-electrolysis-cell produced hydrogen is in fertilizer manufacture. Currently fertilizer is produced in large factories and trucked to farms. With microbial electrolysis cells, very large farms or farm cooperatives could produce hydrogen from wood chips and then through a common process, use the nitrogen in the air to produce ammonia or nitric acid. Both of these are used directly as fertilizer or the ammonia could be used to make ammonium nitrate, sulfate or phosphate. Most fertilizers are currently made from oil, this new system would help reduce use of oil.
I find it pretty interesting that they are able to use commend tools, and easy to find bacteria to do this very interesting process. The electricity could be added from a small solar cell (like the kind in solar toys and calculators). Would everyone have a little hydrogen factory running in the basement? Would you feed it your lawn clippings to power your home? The future is going to be an interesting place.
One thought on “This Is How We Will Make Hydrogen In The Future”
Comments are closed.